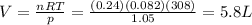Type the correct answer in the box. Express your answer to two significant figures.
A scientis...

Type the correct answer in the box. Express your answer to two significant figures.
A scientist collects 0.24 mole of hydrogen gas in a balloon. The temperature of the hydrogen is 35°C, and the pressure in the
balloon is 1.05 atmospheres. What is the volume of the balloon?
The volume of the balloon is
Niters.
Reset
Next

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Questions

Geography, 17.12.2020 21:10

Biology, 17.12.2020 21:10


Computers and Technology, 17.12.2020 21:10




Mathematics, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10


Mathematics, 17.12.2020 21:10



Mathematics, 17.12.2020 21:10



History, 17.12.2020 21:10

History, 17.12.2020 21:10


Arts, 17.12.2020 21:10

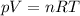
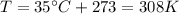 is the temperature of the gas
is the temperature of the gas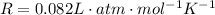 is the gas constant
is the gas constant