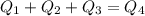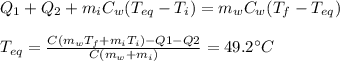Chemistry, 23.02.2020 09:44 dreawongdga
Calculate the final temperature of a mixture of 200.0 g of ice initially at -20.50 °C and 319.0 g of water initially at 91.50 °C.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Calculate the final temperature of a mixture of 200.0 g of ice initially at -20.50 °C and 319.0 g of...
Questions


Social Studies, 20.07.2019 19:00


Social Studies, 20.07.2019 19:00




Health, 20.07.2019 19:00

Mathematics, 20.07.2019 19:00

English, 20.07.2019 19:00

Geography, 20.07.2019 19:00




History, 20.07.2019 19:00

History, 20.07.2019 19:00

History, 20.07.2019 19:00



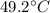
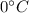 ), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature.
), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature. 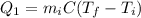
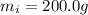 is the mass of the ice
is the mass of the ice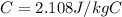 is the specific heat of ice
is the specific heat of ice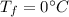 is the final temperature
is the final temperature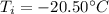 is the initial temperature
is the initial temperature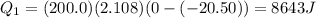
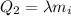
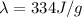 is the specific latent heat of ice
is the specific latent heat of ice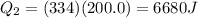
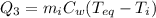
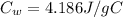 is the specific heat of water
is the specific heat of water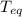 is the equilibrium temperature
is the equilibrium temperature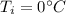 is the initial temperature of ice
is the initial temperature of ice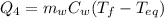
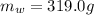 is the mass of the water
is the mass of the water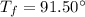 is the initial temperature of the water
is the initial temperature of the water