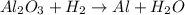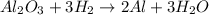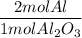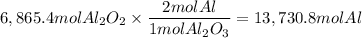Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions



Biology, 17.02.2021 18:00

Mathematics, 17.02.2021 18:00

English, 17.02.2021 18:00




Advanced Placement (AP), 17.02.2021 18:00

English, 17.02.2021 18:00


Mathematics, 17.02.2021 18:00

English, 17.02.2021 18:00

Mathematics, 17.02.2021 18:00


Advanced Placement (AP), 17.02.2021 18:00

Chemistry, 17.02.2021 18:00


Mathematics, 17.02.2021 18:00

Biology, 17.02.2021 18:00