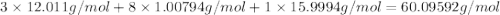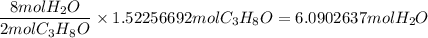Chemistry, 24.02.2020 07:43 bridgettebach
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen gas, how many grams of H2O are formed? MWC = 12.011 g/mol, MWH = 1.00794 g/mol, and MWO = 15.9994 g/mol.
1. 47.9229
2. 84.1255
3. 52.2617
4. 49.8948
5. 86.9152
6. 119.705
7. 88.0758
8. 76.2076
9. 62.9663
10. 109.729

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen...
Questions

Mathematics, 03.06.2020 18:57


Mathematics, 03.06.2020 18:57

Mathematics, 03.06.2020 18:57

Mathematics, 03.06.2020 18:57

History, 03.06.2020 18:57

Physics, 03.06.2020 18:57

Mathematics, 03.06.2020 18:57

History, 03.06.2020 18:57


Biology, 03.06.2020 18:57

Arts, 03.06.2020 18:57

History, 03.06.2020 18:57


Mathematics, 03.06.2020 18:57


French, 03.06.2020 18:57

Mathematics, 03.06.2020 18:57


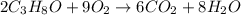
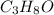 produce 8 moles of
produce 8 moles of 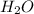 .
.