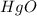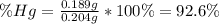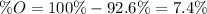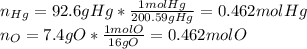Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
An oxide of mercury will thermally decompose when heated. A 0.204 gram sample of the mercury oxide i...
Questions


Mathematics, 04.09.2019 21:30

Social Studies, 04.09.2019 21:30

Biology, 04.09.2019 21:30



Biology, 04.09.2019 21:30

Mathematics, 04.09.2019 21:30

History, 04.09.2019 21:30

Mathematics, 04.09.2019 21:30

Physics, 04.09.2019 21:30

Chemistry, 04.09.2019 21:30



Business, 04.09.2019 21:30