Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
At 380 0C, the equilibrium concentrations are [CH3OH] = 0.20 M, [CO] = 0.35 M, and [H2] = 2.1 M for...
Questions


Computers and Technology, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40



Mathematics, 12.05.2021 21:40



Mathematics, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

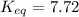
![K_{eq}=\frac{[CO]_{eq}[H_2]^2_{eq}}{[CH_3OH]_{eq}}](/tpl/images/0521/2714/cf196.png)