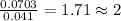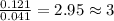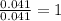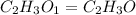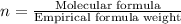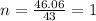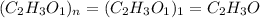Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
A compound is composed of C, H and O. A 1.621 g sample of this compound was combusted, producing 1.9...
Questions





Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00



Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

English, 20.08.2021 01:00





Mathematics, 20.08.2021 01:00

English, 20.08.2021 01:00


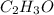
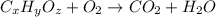
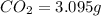
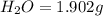
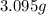 of carbon dioxide,
of carbon dioxide, 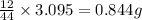 of carbon will be contained.
of carbon will be contained.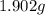 of water,
of water, 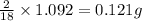 of hydrogen will be contained.
of hydrogen will be contained.![(1.621)-[(0.844)+(0.121)]=0.656g](/tpl/images/0521/3218/1f9d2.png)
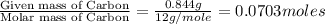
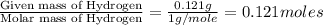
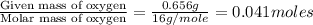
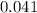 moles.
moles.