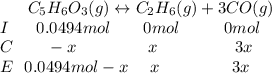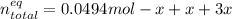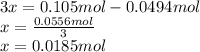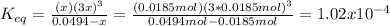Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g sample of pure C5H6O3(g) was placed in an evacuated 2.50 L flask and heated to 200.ºC. At equilibrium, the pressure in the flask was 1.63 atm. Calculate K for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1



Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g...
Questions

Biology, 01.07.2019 10:50



Mathematics, 01.07.2019 10:50

Mathematics, 01.07.2019 10:50

Chemistry, 01.07.2019 10:50



Geography, 01.07.2019 10:50

Spanish, 01.07.2019 10:50

Geography, 01.07.2019 10:50


Physics, 01.07.2019 10:50

Chemistry, 01.07.2019 10:50


Mathematics, 01.07.2019 10:50


Mathematics, 01.07.2019 10:50

History, 01.07.2019 10:50

Mathematics, 01.07.2019 10:50

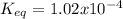
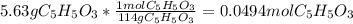
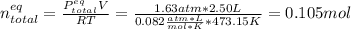
 ", which yields to:
", which yields to: