Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Calculate the pH of a solution that is 0.240 M in sodium formate (HCOONa) and 0.120 M in formic acid...
Questions


Social Studies, 02.12.2020 05:30

Mathematics, 02.12.2020 05:30

Mathematics, 02.12.2020 05:30

English, 02.12.2020 05:30

English, 02.12.2020 05:30

Mathematics, 02.12.2020 05:30




English, 02.12.2020 05:30

Computers and Technology, 02.12.2020 05:30


Engineering, 02.12.2020 05:30

Biology, 02.12.2020 05:30

Mathematics, 02.12.2020 05:30

Health, 02.12.2020 05:30



History, 02.12.2020 05:30

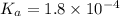
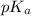 .
.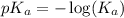
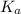 in this expression, we get:
in this expression, we get: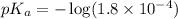
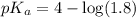
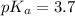
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0521/4495/e961a.png)