
Chemistry, 24.02.2020 19:29 hannahkharel2
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life for the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life f...
Questions

History, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50


English, 01.03.2021 02:50




Mathematics, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50


English, 01.03.2021 02:50


Mathematics, 01.03.2021 02:50



History, 01.03.2021 02:50

Social Studies, 01.03.2021 02:50

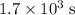 .
. 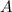 .) The half-life
.) The half-life 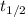 of that first-order reaction would be the time it takes for
of that first-order reaction would be the time it takes for ![[A]](/tpl/images/0521/5010/6aa06.png) (concentration of
(concentration of 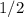 its initial value.
its initial value.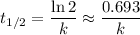 ,
, 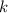 is the rate constant of this reaction.
is the rate constant of this reaction.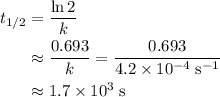 .
.

