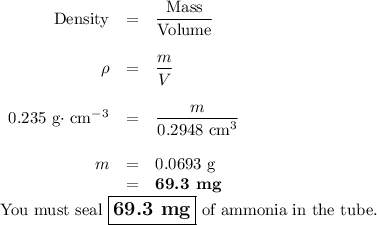
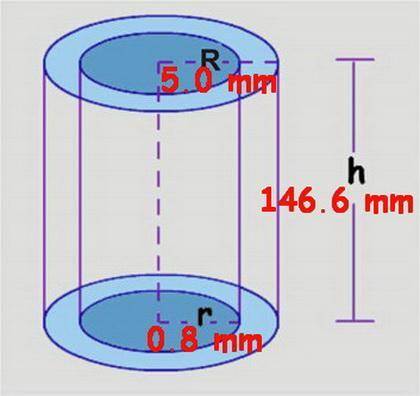
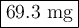At its critical point, ammonia has a density of 0.235 g cm23. You have a special thickwalled glass tube that has a 10.0mm outside diameter, a wall thickness of 4.20 mm, and a length of 155 mm. How much ammonia must you seal into the tube if you wish to observe the disappearance of the meniscus as you heat the tube and its contents to a temperature higher than 132.23°C, the critical temperature?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
At its critical point, ammonia has a density of 0.235 g cm23. You have a special thickwalled glass...
Questions



















Computers and Technology, 17.09.2019 00:00