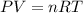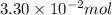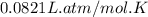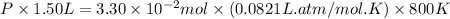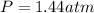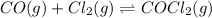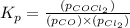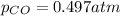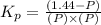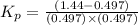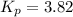Chemistry, 24.02.2020 22:28 pattydixon6
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K, and at equilibrium the pressure of CO was found to be 0.497 atm. Calculate the equilibrium constant, Kp, for the reaction: CO(g) Cl2(g) COCl2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1


Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K...
Questions

Health, 22.09.2019 06:30

Business, 22.09.2019 06:30

English, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30


Health, 22.09.2019 06:30


Mathematics, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30


History, 22.09.2019 06:30



Mathematics, 22.09.2019 06:30


Social Studies, 22.09.2019 06:30


Mathematics, 22.09.2019 06:30


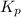 for the reaction is, 3.82
for the reaction is, 3.82