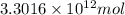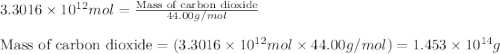Chemistry, 24.02.2020 23:39 mariahrpoulin9630
Assuming gasoline is isooctane, with a density of g/mL, what is the theoretical yield of carbon dioxide produced by the combustion of gal of gasoline (the approximate annual consumption of gasoline in the United States)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Assuming gasoline is isooctane, with a density of g/mL, what is the theoretical yield of carbon diox...
Questions

Biology, 18.02.2020 17:52


Mathematics, 18.02.2020 17:52





Mathematics, 18.02.2020 17:52



Mathematics, 18.02.2020 17:52



Computers and Technology, 18.02.2020 17:52

History, 18.02.2020 17:52


Computers and Technology, 18.02.2020 17:52



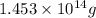
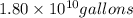
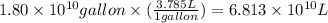
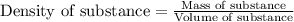
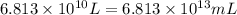 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)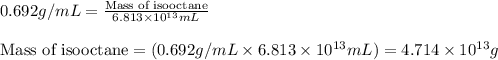
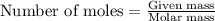 .....(1)
.....(1)
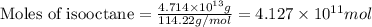
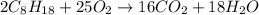
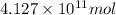 of isooctane will produce =
of isooctane will produce = 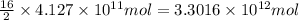 of carbon dioxide
of carbon dioxide