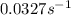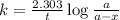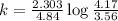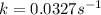Chemistry, 24.02.2020 23:41 davisbrittany5784
Consider the following first-order reaction: A → B. The concentration of A at the start of the reaction is 4.17 M and after 4.84 s is 3.56 M. (a) Using the integrated rate law for a first-order reaction, calculate the value of the rate constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3


Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Consider the following first-order reaction: A → B. The concentration of A at the start of the react...
Questions


Mathematics, 30.08.2021 20:10



Mathematics, 30.08.2021 20:10



Mathematics, 30.08.2021 20:10



Geography, 30.08.2021 20:10

Biology, 30.08.2021 20:10



Mathematics, 30.08.2021 20:10

Mathematics, 30.08.2021 20:10




Mathematics, 30.08.2021 20:10