
Chemistry, 25.02.2020 00:38 alinadancer2717
If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4 mol L−1 s−1, what is the rate of appearance of Br2(aq) at that moment?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4 mol...
Questions




Mathematics, 30.01.2020 22:03


English, 30.01.2020 22:03

Chemistry, 30.01.2020 22:03


Mathematics, 30.01.2020 22:03

Computers and Technology, 30.01.2020 22:03



English, 30.01.2020 22:03






Mathematics, 30.01.2020 22:03

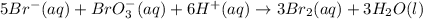
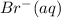 at a particular moment during the reaction is
at a particular moment during the reaction is 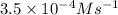 , what is the rate of appearance of Br₂(aq) at that moment?
, what is the rate of appearance of Br₂(aq) at that moment?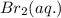 for the reaction is
for the reaction is 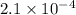
![Br^-(aq.)=-\frac{d[Br^-]}{dt}=3.5\times 10^{-4}Ms^{-1}](/tpl/images/0522/2019/1c6cc.png)
![\text{Rate of appearance of }Br_2=+\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0522/2019/1ea6a.png)
![\frac{1}{5}\frac{d[Br^-]}{dt}=\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0522/2019/30b24.png)
![\frac{1}{5}\times (3.5\times 10^{-4})=\frac{1}{3}\frac{d[Br_2]}{dt}\\\\\frac{d[Br_2]}{dt}=\frac{3}{5}\times (3.5\times 10^{-4})=2.1\times 10^{-4}](/tpl/images/0522/2019/82270.png)


