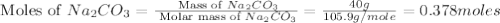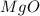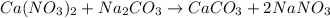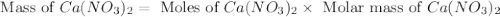Chemistry, 25.02.2020 00:41 melissareid65
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at what point the masses of the two reactants react "evenly." That is, how many grams of Ca(NO3)2 must be added to just consume the 40 g Na2CO3 initially available?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

You know the right answer?
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at...
Questions

Social Studies, 05.05.2021 23:40

Mathematics, 05.05.2021 23:40


Mathematics, 05.05.2021 23:40

World Languages, 05.05.2021 23:40


Mathematics, 05.05.2021 23:40


Mathematics, 05.05.2021 23:40




Physics, 05.05.2021 23:40


Mathematics, 05.05.2021 23:40

History, 05.05.2021 23:40

Chemistry, 05.05.2021 23:40



English, 05.05.2021 23:40

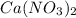 added must be, 61.9 grams
added must be, 61.9 grams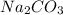 = 40 g
= 40 g