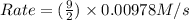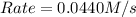The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the initial rate be if [ A ] is halved and [ B ] is tripled? initial rate: .00978 M / s What will the initial rate be if [ A ] is tripled and [ B ] is halved? initial rate:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the...
Questions

Physics, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30


Mathematics, 24.09.2019 00:30



History, 24.09.2019 00:30

Advanced Placement (AP), 24.09.2019 00:30

Business, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30

Mathematics, 24.09.2019 00:30


Social Studies, 24.09.2019 00:30




Health, 24.09.2019 00:30

![Rate=k[A][B]^2](/tpl/images/0522/4296/d8f90.png)
![Rate=k\times (\frac{[A]}{2})\times (3\times [B])^2](/tpl/images/0522/4296/31c7e.png)
![Rate=k\times (\frac{[A]}{2})\times 9\times [B]^2](/tpl/images/0522/4296/e768d.png)
![Rate=k\times (\frac{9}{2})\times [A]\times [B]^2](/tpl/images/0522/4296/4e890.png)
![k[A][B]^2](/tpl/images/0522/4296/8ef61.png) = 0.0440 M/s
= 0.0440 M/s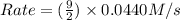
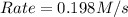
![Rate=k\times (\frac{[B]}{2})\times (3\times [A])^2](/tpl/images/0522/4296/e26df.png)
![Rate=k\times (\frac{[B]}{2})\times 9\times [A]^2](/tpl/images/0522/4296/e83c0.png)