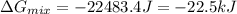Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A sample containing 3.25 moles of He is mixed with 2.25 moles of Ne and 1.75 moles of Ar. All compon...
Questions

Social Studies, 11.09.2021 01:00


History, 11.09.2021 01:00


History, 11.09.2021 01:00




Mathematics, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00


English, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00

Chemistry, 11.09.2021 01:00

English, 11.09.2021 01:00



Mathematics, 11.09.2021 01:00


Chemistry, 11.09.2021 01:00

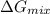 is, -22.5 kJ
is, -22.5 kJ![\Delta G_{mix}=nRT\times [X_{He}\ln (X_{He})+X_{Ne}\ln (X_{Ne})+X_{Ar}\ln (X_{Ar})]](/tpl/images/0522/4111/f4a32.png)
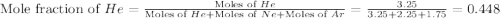
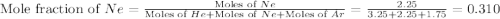
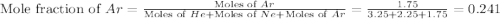
![\Delta G_{mix}=(7.25mol)\times (8.314J/mol.K)\times (350K)\times [0.448\ln (0.448)+0.310\ln (0.310)+0.241\ln (0.241)]](/tpl/images/0522/4111/274c3.png)