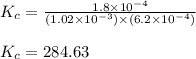Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Consider the following reaction: Fe3+(aq)+SCN−(aq)⇌FeSCN2+(aq) A solution is made containing an init...
Questions

Mathematics, 10.07.2021 23:00




Mathematics, 10.07.2021 23:00


Mathematics, 10.07.2021 23:00

Mathematics, 10.07.2021 23:00

Mathematics, 10.07.2021 23:00




Mathematics, 10.07.2021 23:00



Mathematics, 10.07.2021 23:30

Mathematics, 10.07.2021 23:30


Mathematics, 10.07.2021 23:30

Mathematics, 10.07.2021 23:30

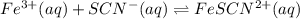 A solution is made containing an initial
A solution is made containing an initial ![[Fe^{3+}]](/tpl/images/0522/5263/5ed68.png) of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.
of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.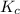 for above equation is 284.63
for above equation is 284.63![[Fe^{3+}]=1.2\times 10^{-3}M](/tpl/images/0522/5263/e152e.png)
![[SCN^{-}]=8.0\times 10^{-4}M](/tpl/images/0522/5263/5f5c1.png)
![[FeSCN^{2+}]=1.8\times 10^{-4}M](/tpl/images/0522/5263/2496b.png)
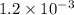
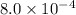
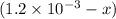
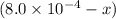 x
x![[Fe^{3+}]=(1.2\times 10^{-3})-(1.8\times 10^{-4)=1.02\times 10^{-3}M](/tpl/images/0522/5263/a018d.png)
![[SCN^{-}]=(8.0\times 10^{-3})-(1.8\times 10^{-4)=6.2\times 10^{-4}M](/tpl/images/0522/5263/febab.png)
![K_c=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0522/5263/417f5.png)