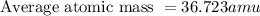Chemistry, 25.02.2020 03:05 cecilf57p2rpuf
On a mission to a newly discovered planet, an astronaut finds chlorine abundances of 13.85 % for 35Cl and 86.15 % for 37Cl. What is the atomic mass of chlorine for this location?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
On a mission to a newly discovered planet, an astronaut finds chlorine abundances of 13.85 % for 35C...
Questions







Mathematics, 30.12.2020 22:10

English, 30.12.2020 22:10

English, 30.12.2020 22:10


Computers and Technology, 30.12.2020 22:10

Mathematics, 30.12.2020 22:10

Mathematics, 30.12.2020 22:10



Mathematics, 30.12.2020 22:10

Mathematics, 30.12.2020 22:10


Mathematics, 30.12.2020 22:10


![\text{Average atomic mass }=[(35\times 0.1385)+(37\times 0.8615)]](/tpl/images/0522/6204/3c4d5.png)