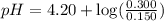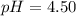Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
Calculate the pH of a solution prepared by dissolving 0.150 mol of benzoic acid and 0.300 mol of sod...
Questions

Biology, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00

History, 13.12.2021 05:00


Mathematics, 13.12.2021 05:00

English, 13.12.2021 05:00

English, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00


Mathematics, 13.12.2021 05:00






Mathematics, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00

Mathematics, 13.12.2021 05:00

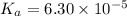
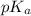 .
.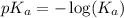
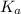 in this expression, we get:
in this expression, we get: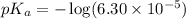
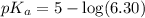
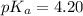
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0522/6376/e961a.png)