
Chemistry, 25.02.2020 03:23 tdyson3p6xvtu
Calculate the standard heat of reaction for the following methane-generating reaction of methanogenic bacteria: 4CH3NH2(g) + 2H2O(l) → 3CH4(g) + CO2(g) + 4NH3(g) Given that ΔHfo(CH3NH2, g) = –22.97 kJ/mol; ΔHfo(H2O, l) = –285.8 kJ/mol; ΔHfo(CH4, g) = –74.8 kJ/mol; ΔHfo(CO2, g) = –393.5 kJ/mol ΔHfo(NH3, g) = –46.1 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Calculate the standard heat of reaction for the following methane-generating reaction of methanogeni...
Questions




Social Studies, 07.07.2019 07:30



History, 07.07.2019 07:30

Mathematics, 07.07.2019 07:30

Biology, 07.07.2019 07:30


Mathematics, 07.07.2019 07:30

Biology, 07.07.2019 07:30

History, 07.07.2019 07:30





English, 07.07.2019 07:30

English, 07.07.2019 07:30

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0522/6322/e893d.png)
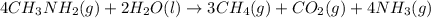
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CH_4(g))})+(1\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(NH_3(g))})]-[(4\times \Delta H_f_{(CH_3NH_2(g))})+(2\times \Delta H_f_{(H_2O(l))})]](/tpl/images/0522/6322/6730f.png)
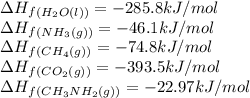
![\Delta H_{rxn}=[(3\times (-74.8))+(1\times (-393.5))+(4\times (-46.1))]-[(4\times (-22.97))+(2\times (-285.8))]\\\\\Delta H_{rxn}=-138.82kJ](/tpl/images/0522/6322/fdc01.png)


