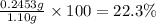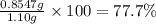Chemistry, 25.02.2020 03:23 alizeleach0123
>A 1.10-g sample contains only glucose (C6H12O6) and sucrose (C12H22O11). When a sample is dissolved in water to a total solution volume of 25.0 mL, the osmotic pressure of the solution is 3.78 atm at 298 K. What is the percent mass of the glucose in the sample, and what is the percent mass of sucrose in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
>A 1.10-g sample contains only glucose (C6H12O6) and sucrose (C12H22O11). When a sample is dissol...
Questions



Computers and Technology, 18.10.2021 17:40

Advanced Placement (AP), 18.10.2021 17:40

World Languages, 18.10.2021 17:40


Social Studies, 18.10.2021 17:40

Mathematics, 18.10.2021 17:40


History, 18.10.2021 17:40


Geography, 18.10.2021 17:40

Mathematics, 18.10.2021 17:40

English, 18.10.2021 17:40

Law, 18.10.2021 17:40

Mathematics, 18.10.2021 17:40



English, 18.10.2021 17:40

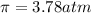
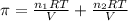
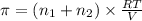
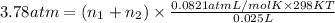
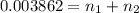
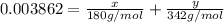
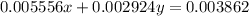 ..[2]
..[2]