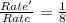Chemistry, 25.02.2020 04:05 alialbinn6969
The gas phase reaction between NO and O2 is second order with respect to NO and first order with respect to 02, what would happen to the reaction rate if the concentrations of both reactants were halved with everything else held constant?
a. It would decrease by a factor of 8 It would remain unchanged.
b. It would decrease by a factor of 16.
c. It would decrease by a factor of 4.
d. It would decrease by a factor of 2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
The gas phase reaction between NO and O2 is second order with respect to NO and first order with res...
Questions


Social Studies, 24.08.2021 14:00

History, 24.08.2021 14:00

Chemistry, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

Medicine, 24.08.2021 14:00



Social Studies, 24.08.2021 14:00

Law, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

English, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

Geography, 24.08.2021 14:00

Geography, 24.08.2021 14:00


Biology, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

Mathematics, 24.08.2021 14:00

![Rate=k[NO]^2[O_2]^1](/tpl/images/0522/7517/1df0d.png) (1)
(1)![Rate'=k{\frac{[NO]}{2}^2}{\frac{[O_2]}{2}^1}](/tpl/images/0522/7517/19c10.png) (2)
(2)![\frac{Rate'}{Rate}=\frac{k\frac{[NO]}{2}^2\times \frac{([O_2]}{2})^1}{k[NO]^2[O_2]^1}](/tpl/images/0522/7517/9c613.png)