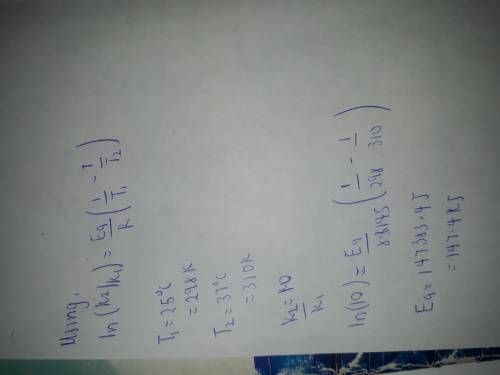Chemistry, 25.02.2020 04:33 floressavanna15
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rate constant of a reaction by ten-fold, what is the value for the activation energy of the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rat...
Questions




Mathematics, 17.04.2021 18:30








Mathematics, 17.04.2021 18:30


Mathematics, 17.04.2021 18:30