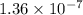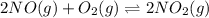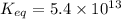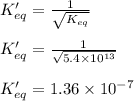Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2


You know the right answer?
The Keq for the equilibrium below is 5.4 × 1013 at 480.0 °C. 2NO (g) + O2 (g) 2NO2 (g) What is the v...
Questions


Mathematics, 28.05.2021 02:20






History, 28.05.2021 02:20


Mathematics, 28.05.2021 02:20

Mathematics, 28.05.2021 02:20

Mathematics, 28.05.2021 02:20

Social Studies, 28.05.2021 02:20

Mathematics, 28.05.2021 02:20

Mathematics, 28.05.2021 02:20




Mathematics, 28.05.2021 02:20

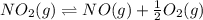 equation is
equation is