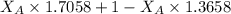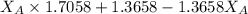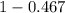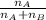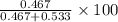A student was trying to determine the mole percent of A in a mixture of A and B using refractive index. If their mixture has a refractive index of 1.5248 and pure A and pure B each had refractive indices of 1.7058 and 1.3658, respectively, what was the mole percent of A in their mixture. Type your numerical answer rounded to the 3rd decimal place (i. e. 45.982 or 9.550, etc) without a percent sign.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

You know the right answer?
A student was trying to determine the mole percent of A in a mixture of A and B using refractive ind...
Questions


Health, 29.07.2019 01:30






Social Studies, 29.07.2019 01:30


World Languages, 29.07.2019 01:30



SAT, 29.07.2019 01:30


Mathematics, 29.07.2019 01:30



Mathematics, 29.07.2019 01:30

Computers and Technology, 29.07.2019 01:30

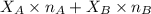
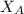 = mole fraction of A
= mole fraction of A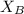 = mole fraction of B
= mole fraction of B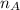 = refractive index of A
= refractive index of A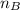 = refractive index of B
= refractive index of B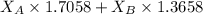 ........ (1)
........ (1)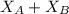 = 1
= 1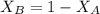 ......... (2)
......... (2)