Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
The heat of fusion of acetone is 5.7 kJ/mol. Calculate to two significant figures the entropy change...
Questions


Mathematics, 26.01.2021 14:50

Mathematics, 26.01.2021 14:50

Physics, 26.01.2021 14:50


Mathematics, 26.01.2021 14:50

Mathematics, 26.01.2021 14:50


Mathematics, 26.01.2021 14:50

Spanish, 26.01.2021 14:50


Mathematics, 26.01.2021 14:50



Chemistry, 26.01.2021 14:50


Mathematics, 26.01.2021 14:50



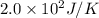
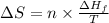
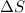 = Entropy change
= Entropy change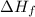 = enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)![-94.7^oC=[273-94.7]=178.3K](/tpl/images/0522/9891/25895.png)