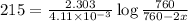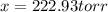Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81 h at 25 ∘C. If a 1.7 −L reaction vessel initially contains 755 torr of N2O5 at 25 ∘C, what partial pressure of O2 will be present in the vessel after 215 minutes?

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

You know the right answer?
Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reacti...
Questions

Biology, 10.03.2021 14:00


English, 10.03.2021 14:00


Mathematics, 10.03.2021 14:00


Chemistry, 10.03.2021 14:00

History, 10.03.2021 14:00

Health, 10.03.2021 14:00

English, 10.03.2021 14:00



Mathematics, 10.03.2021 14:00

Chemistry, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Health, 10.03.2021 14:00

Geography, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00


Health, 10.03.2021 14:00

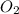 is, 222.93 torr
is, 222.93 torr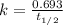
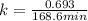
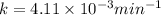
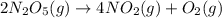
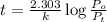
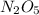 = 760 torr
= 760 torr