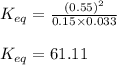Chemistry, 25.02.2020 19:21 vazquezemmy8
Consider the following chemical reaction: H2 (g) + I2 (g) 2HI (g) At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were and respectively. The value of Keq for this reaction is.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3


Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Consider the following chemical reaction: H2 (g) + I2 (g) 2HI (g) At equilibrium in a particular exp...
Questions


Geography, 21.01.2021 08:10


Mathematics, 21.01.2021 08:10



Mathematics, 21.01.2021 08:20

Advanced Placement (AP), 21.01.2021 08:20

Biology, 21.01.2021 08:20

English, 21.01.2021 08:20

Chemistry, 21.01.2021 08:20


Mathematics, 21.01.2021 08:20


Geography, 21.01.2021 08:20





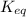 for the given reaction is 61.11
for the given reaction is 61.11 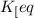
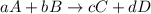
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0523/4527/9c8b0.png)
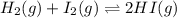
![K_{eq}=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0523/4527/ad3b5.png)
![[HI]_{eq}=0.55M](/tpl/images/0523/4527/ad244.png)
![[H_2]_{eq}=0.15M](/tpl/images/0523/4527/4357e.png)
![[I_2]_{eq}=0.033M](/tpl/images/0523/4527/b80ab.png)