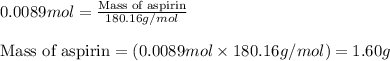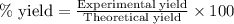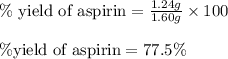Chemistry, 25.02.2020 19:53 chloegrace359
Aspirin can be made in the laboratory by reacting acetic anhydride (C 4H 6O 3) with salicylic acid (C 7H 6O 3) to form aspirin (C 9H 8O 4) and acetic acid (C 2H 4O 2). The balanced equation is
C 4H 6O 3+C 7H 6O 3?C 9H 8O 4+C 2H 4O 2
In a laboratory synthesis, a student begins with 2.80mL of acetic anhydride (density=1.08g/ml) and 1.24g of salicylic acid. Once the reaction is complete, the student collects 1.24g of aspirin.
Determine the limiting reactant for the reaction.
Determine the theoretical yield of aspirin for the reaction.
Determine the percent yield of aspirin for the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Aspirin can be made in the laboratory by reacting acetic anhydride (C 4H 6O 3) with salicylic acid (...
Questions

English, 10.11.2020 18:30

Biology, 10.11.2020 18:30

Biology, 10.11.2020 18:30



Biology, 10.11.2020 18:30


Mathematics, 10.11.2020 18:30

Mathematics, 10.11.2020 18:30

Mathematics, 10.11.2020 18:30



Mathematics, 10.11.2020 18:30

Mathematics, 10.11.2020 18:30




Mathematics, 10.11.2020 18:30

English, 10.11.2020 18:30

Mathematics, 10.11.2020 18:30

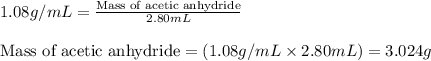
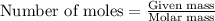 .....(1)
.....(1)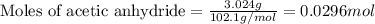
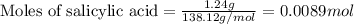
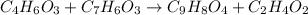
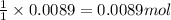 of acetic anhydride
of acetic anhydride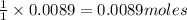 of aspirin
of aspirin