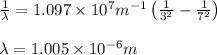Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
Consider a transition of the electron in the hydrogen atom from n=3 to n=7.
a) Determine...
a) Determine...
Questions

Mathematics, 16.12.2020 21:20





Mathematics, 16.12.2020 21:20

Advanced Placement (AP), 16.12.2020 21:20

Mathematics, 16.12.2020 21:20



Mathematics, 16.12.2020 21:20


Chemistry, 16.12.2020 21:20

English, 16.12.2020 21:20



Mathematics, 16.12.2020 21:20


Chemistry, 16.12.2020 21:20

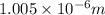
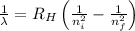
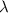 = Wavelength of radiation
= Wavelength of radiation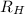 = Rydberg's Constant =
= Rydberg's Constant = 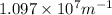
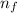 = Higher energy level = 7
= Higher energy level = 7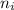 = Lower energy level = 3
= Lower energy level = 3