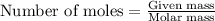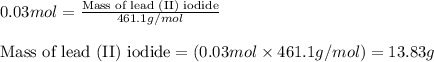Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include ph...
Questions


Mathematics, 19.02.2021 19:10

Computers and Technology, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


History, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10

Chemistry, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Social Studies, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10


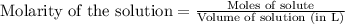
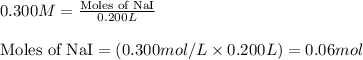
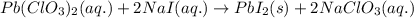
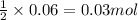 of lead (II) iodide
of lead (II) iodide