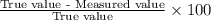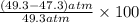Chemistry, 25.02.2020 21:59 sierram298
In Part B the given conditions were 1.00 mol of argon in a 0.500-L container at 27.0 ∘C . You identified that the ideal pressure (Pideal) was 49.3 atm , and the real pressure (Preal) was 47.3 atm under these conditions. The percent difference between the ideal and real gas is .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
In Part B the given conditions were 1.00 mol of argon in a 0.500-L container at 27.0 ∘C . You identi...
Questions

Mathematics, 01.10.2019 18:30

English, 01.10.2019 18:30



English, 01.10.2019 18:30

Social Studies, 01.10.2019 18:30


Biology, 01.10.2019 18:30


Mathematics, 01.10.2019 18:30

History, 01.10.2019 18:30

Mathematics, 01.10.2019 18:30

English, 01.10.2019 18:30


History, 01.10.2019 18:30

Social Studies, 01.10.2019 18:30

Mathematics, 01.10.2019 18:30