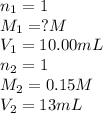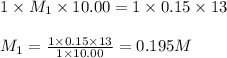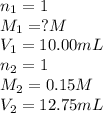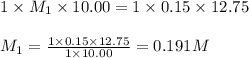Chemistry, 25.02.2020 22:16 kaylailkanic1487
For each of your NCount ACCEPTABLE trials enter the values you calculated for the molarity of your HCl solution in the same sequence corresponding to the equivalence point volumes. You should report the molarities to 4 significant figures, e. g. 0.2314 M.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
For each of your NCount ACCEPTABLE trials enter the values you calculated for the molarity of your H...
Questions




Biology, 26.09.2019 23:00
















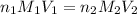 ......(1)
......(1)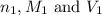 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl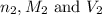 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.