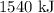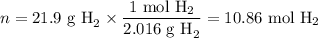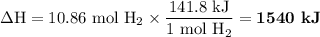Chemistry, 25.02.2020 22:09 lucyamine0
When 1 mole of hydrogen gas (H2) reacts with excess oxygen to form water at a constant pressure, 241.8 KJ of energy is released as heat. Calculate ΔH for a process in which 21.9 g sample of hydrogen gas (H2) reacts with excess oxygen at constant pressure.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
When 1 mole of hydrogen gas (H2) reacts with excess oxygen to form water at a constant pressure, 241...
Questions


Health, 12.08.2020 07:01



Health, 12.08.2020 07:01




Mathematics, 12.08.2020 07:01



Computers and Technology, 12.08.2020 07:01

English, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01


Social Studies, 12.08.2020 07:01