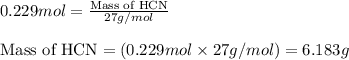Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.90 g NH3, 11.0 g O2, and 4.67 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process...
Questions


Mathematics, 20.08.2021 16:00

History, 20.08.2021 16:00

Mathematics, 20.08.2021 16:00







Mathematics, 20.08.2021 16:00

Mathematics, 20.08.2021 16:00

History, 20.08.2021 16:00


Mathematics, 20.08.2021 16:00

History, 20.08.2021 16:00

Mathematics, 20.08.2021 16:00

Chemistry, 20.08.2021 16:00

History, 20.08.2021 16:00

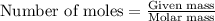 .....(1)
.....(1)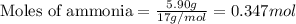
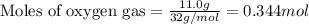
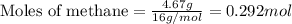
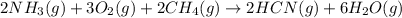
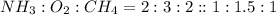
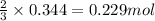 of HCN
of HCN