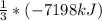Chemistry, 25.02.2020 22:49 alicciardone01
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 moles of NH4NO3. What is the enthalpy change for 1.0 mole of NH4NO3 in this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1


Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

You know the right answer?
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 mole...
Questions

Mathematics, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30

Health, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30


Biology, 21.05.2021 16:30


Mathematics, 21.05.2021 16:30

English, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30


Mathematics, 21.05.2021 16:30

Social Studies, 21.05.2021 16:30





Biology, 21.05.2021 16:40


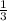 of the total enthaply of the 3 moles
of the total enthaply of the 3 moles