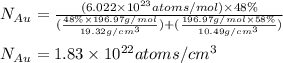Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 42 wt% Au and 58 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3, respectively. The atomic weight of Au is 196.97 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic c...
Questions



Arts, 07.04.2021 23:30


Mathematics, 07.04.2021 23:30


History, 07.04.2021 23:30


Mathematics, 07.04.2021 23:30

Chemistry, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30

Social Studies, 07.04.2021 23:30



Mathematics, 07.04.2021 23:30

Physics, 07.04.2021 23:30



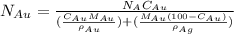
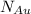 = number of gold atoms per cubic centimeters
= number of gold atoms per cubic centimeters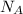 = Avogadro's number =
= Avogadro's number = 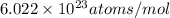
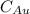 = Mass percent of gold in the alloy = 42 %
= Mass percent of gold in the alloy = 42 %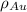 = Density of pure gold =
= Density of pure gold = 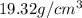
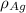 = Density of pure silver =
= Density of pure silver = 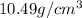
 = molar mass of gold = 196.97 g/mol
= molar mass of gold = 196.97 g/mol