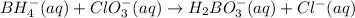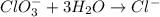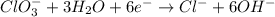Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the states of all reactants and products in your balanced equation. You do not need to include the states with the identities of the oxidizing and reducing agents.
BH4−(aq) + ClO3−(aq) → H2BO3−(aq) + Cl−(aq) [basic]
a. The oxidizing agent is:
b. The reducing agent is:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1


Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the...
Questions

Mathematics, 17.06.2021 03:50


English, 17.06.2021 03:50


History, 17.06.2021 03:50

Mathematics, 17.06.2021 03:50

Mathematics, 17.06.2021 04:00




Mathematics, 17.06.2021 04:00

Mathematics, 17.06.2021 04:00

Social Studies, 17.06.2021 04:00

Mathematics, 17.06.2021 04:00

Mathematics, 17.06.2021 04:00

Mathematics, 17.06.2021 04:00

Health, 17.06.2021 04:00