Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand fo...

Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand for the rate constant of this reaction, and k-1 stand for the rate constant of the reverse reaction. write an expression that gives the equilibrium concentration of N2O in terms of k1, k-1 and the equilibrium concentrations of N2 and O

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Questions



History, 14.07.2019 22:30




Mathematics, 14.07.2019 22:30


Mathematics, 14.07.2019 22:30





Biology, 14.07.2019 22:30

Mathematics, 14.07.2019 22:30

Mathematics, 14.07.2019 22:30



English, 14.07.2019 22:30

History, 14.07.2019 22:30

![[N_2O]=\frac{K^{-1}}{K_1}\times [N_2][O_2]](/tpl/images/0524/0986/7a4ba.png)
![R_f=K_1\times [N_2O]](/tpl/images/0524/0986/5d806.png)
![R_b=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/327e3.png)
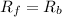
![K_1\times [N_2O]=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/21d13.png)


