
Chemistry, 26.02.2020 01:54 xxtonixwilsonxx
Consider the following system at equilibrium:A(aq)+B(aq) <---> 2C(aq)Classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the net reaction.1. Increase A- Right2. Increase B- Right3. Increase C- Left4. Decrease A- Right5. Decrease B- Right6. Decrease C- lEft7. Double A and Halve B- NO Shift8. Double both B and C- NO shift

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
Consider the following system at equilibrium:A(aq)+B(aq) <---> 2C(aq)Classify each of the foll...
Questions


English, 24.08.2019 11:30



Biology, 24.08.2019 11:30

Chemistry, 24.08.2019 11:30

Biology, 24.08.2019 11:30

Social Studies, 24.08.2019 11:30


Biology, 24.08.2019 11:30

Physics, 24.08.2019 11:30

Mathematics, 24.08.2019 11:30

Health, 24.08.2019 11:30



Computers and Technology, 24.08.2019 11:30

Chemistry, 24.08.2019 11:30

Chemistry, 24.08.2019 11:30

English, 24.08.2019 11:30

History, 24.08.2019 11:30

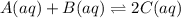
![K=\frac{[C]^2}{[A][B]}](/tpl/images/0524/2481/477c9.png)
![K'=\frac{[C]^2}{[2A][\frac{B}{2}]}=\frac{[C]^2}{[A][B]}](/tpl/images/0524/2481/7596a.png)
![K'=\frac{[2C]^2}{[A][2B]}=\frac{4[C]^2}{[A][2B]}=\frac{2[C]^2}{[A][B]}](/tpl/images/0524/2481/c241a.png)


