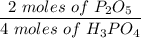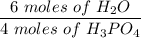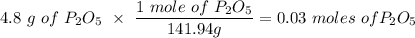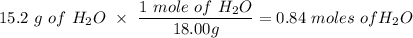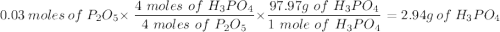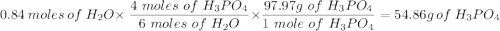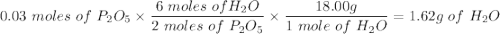Chemistry, 26.02.2020 02:54 preciousharrington13
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and 15.2 grams H2O, what will be your limiting reactant? How many grams of H3PO4 are produced?
Whenever I multiply P2O5 I always get 3.3 grams rather than 6.6 grams. However, when I multiply H2O I get the correct answer.
How many grams of the excess reagent remain unreacted?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and...
If you begin with 4.8 grams of P205 and...
Questions







Biology, 21.03.2020 08:04

Mathematics, 21.03.2020 08:04

Computers and Technology, 21.03.2020 08:04

Mathematics, 21.03.2020 08:04






History, 21.03.2020 08:04


Chemistry, 21.03.2020 08:04