
Chemistry, 26.02.2020 16:25 michaela134
A mixture of 0.439 M H 2 , 0.317 M I 2 , and 0.877 M HI is enclosed in a vessel and heated to 430 °C. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 54.3 at 430 ∘ C Calculate the equilibrium concentrations of each gas at 430 ∘ C .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
A mixture of 0.439 M H 2 , 0.317 M I 2 , and 0.877 M HI is enclosed in a vessel and heated to 430 °C...
Questions

Mathematics, 29.11.2020 15:20

Mathematics, 29.11.2020 15:20












Social Studies, 29.11.2020 15:20

Mathematics, 29.11.2020 15:20

Mathematics, 29.11.2020 15:20

History, 29.11.2020 15:20

Chemistry, 29.11.2020 15:20


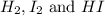 at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.
at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.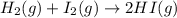
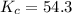
![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0524/8903/8a740.png)
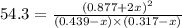
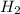 at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M
at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M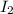 at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M
at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M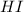 at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M
at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M

