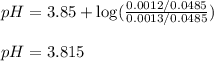Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
A student performs a titration of 25.0 mL of 0.100 M lactic acid (HC3H5O3), using 0.050 M sodium hyd...
Questions


Mathematics, 31.01.2020 10:45


History, 31.01.2020 10:45





History, 31.01.2020 10:45

Social Studies, 31.01.2020 10:45


Arts, 31.01.2020 10:45

Advanced Placement (AP), 31.01.2020 10:45

English, 31.01.2020 10:45

Social Studies, 31.01.2020 10:45

Mathematics, 31.01.2020 10:45



Mathematics, 31.01.2020 10:45

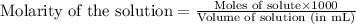
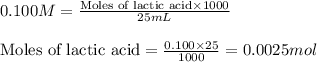
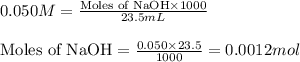
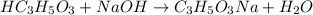
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0524/8883/e4eea.png)
![pH=pK_a+\log(\frac{[C_3H_5O_3Na]}{[HC_3H_5O_3]})](/tpl/images/0524/8883/28764.png)
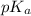 = negative logarithm of acid dissociation constant of lactic acid = 3.85
= negative logarithm of acid dissociation constant of lactic acid = 3.85![[HC_3H_5O_3]=\frac{0.0013}{0.0485}](/tpl/images/0524/8883/28573.png)
![[C_3H_5O_3Na]=\frac{0.0012}{0.0485}](/tpl/images/0524/8883/f1a5a.png)