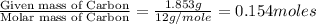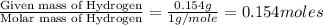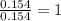Chemistry, 26.02.2020 16:40 kayla942783
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is the empirical formula of the compound? a. CH2 b. C2Hw c. C3H4 d. CH e. C5H12

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2


Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1
You know the right answer?
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is...
Questions

Mathematics, 05.12.2019 04:31

Social Studies, 05.12.2019 04:31

Chemistry, 05.12.2019 04:31


Mathematics, 05.12.2019 04:31


Social Studies, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Biology, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

History, 05.12.2019 04:31

History, 05.12.2019 04:31




Computers and Technology, 05.12.2019 04:31

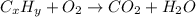
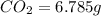
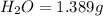
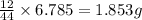 of carbon will be contained.
of carbon will be contained.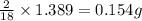 of hydrogen will be contained.
of hydrogen will be contained.