Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
The rate constants of some reactions double with every 10 degree rise in temperature. Assume that a...
Questions

Mathematics, 05.02.2021 06:00



Biology, 05.02.2021 06:00

Mathematics, 05.02.2021 06:00


Mathematics, 05.02.2021 06:00

Mathematics, 05.02.2021 06:00

Mathematics, 05.02.2021 06:00

History, 05.02.2021 06:00


English, 05.02.2021 06:00

Chemistry, 05.02.2021 06:00

History, 05.02.2021 06:00

Mathematics, 05.02.2021 06:00





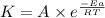
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0524/9978/6d953.png)
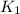 = rate constant at 271 K
= rate constant at 271 K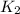 = rate constant at 281 K =
= rate constant at 281 K = 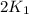
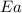 = activation energy for the reaction = ?
= activation energy for the reaction = ?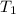 = initial temperature = 271 K
= initial temperature = 271 K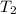 = final temperature = 281 K
= final temperature = 281 K![\log (\frac{2K_1}{K_1})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{271K}-\frac{1}{281K}]](/tpl/images/0524/9978/ceafb.png)