
Chemistry, 26.02.2020 17:47 clairebear66
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the rate of formation of O2 is 7.78 x 10-1 M/s, what is the rate of the loss of O3? 2 O3(g) → 3 O2(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the r...
Questions

Mathematics, 09.07.2019 16:00

Mathematics, 09.07.2019 16:00

Mathematics, 09.07.2019 16:00


Mathematics, 09.07.2019 16:00



Mathematics, 09.07.2019 16:00



Mathematics, 09.07.2019 16:00


History, 09.07.2019 16:00


Mathematics, 09.07.2019 16:00

Chemistry, 09.07.2019 16:00




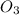 is 0.52M/s
is 0.52M/s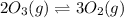
![-\frac{1d[O_3]}{2dt}](/tpl/images/0525/0164/0b459.png)
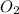 =
=![+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/4dcb2.png)
![-\frac{1d[O_3]}{2dt}=+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/b3aa3.png)
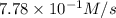
![\frac{2d[O_2]}{3dt}=\frac{2}{3}\times 7.78\times 10^{-1}M/s=0.52M/s](/tpl/images/0525/0164/964d0.png)



