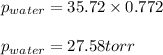Chemistry, 26.02.2020 20:52 cocomorillo35181
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. Using the Clausius-Clapeyron equation and the boiling point of water as 100.0°C at 760 torr, calculate the vapor pressure (in torr) of water in the air. Use 40.7 kJ/mol as the ∆Hvap of water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. U...
Questions


English, 06.04.2021 22:00

Mathematics, 06.04.2021 22:00



Mathematics, 06.04.2021 22:00

Mathematics, 06.04.2021 22:00

Mathematics, 06.04.2021 22:00





History, 06.04.2021 22:00

Mathematics, 06.04.2021 22:00



Mathematics, 06.04.2021 22:00


Mathematics, 06.04.2021 22:00

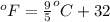
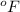 = temperature in Fahrenheit
= temperature in Fahrenheit 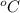 = temperature in centigrade
= temperature in centigrade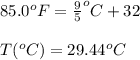
![\ln(\frac{P_2}{P_1})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/3984/5c76e.png)
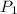 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr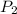 = final pressure = ?
= final pressure = ?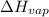 = Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)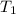 = initial temperature =
= initial temperature = ![100^oC=[100+273]K=373K](/tpl/images/0525/3984/44e24.png)
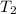 = final temperature =
= final temperature = ![29.44^oC=[29.44+273]=302.44K](/tpl/images/0525/3984/ddd83.png)
![\ln(\frac{P_2}{760})=\frac{40700J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{302.44}]\\\\P_2=35.72torr](/tpl/images/0525/3984/e0926.png)
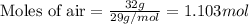
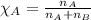

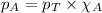
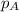 = vapor pressure of water = ?
= vapor pressure of water = ?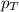 = total pressure = 35.72 torr
= total pressure = 35.72 torr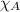 = mole fraction of water = 0.774
= mole fraction of water = 0.774