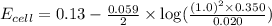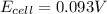Chemistry, 26.02.2020 20:54 adazeb2003
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1.00 M) || Sn2+ (aq, 0.350 M) | Sn4+ (aq, 0.020 M) | Pt (s)The standard reduction potentials are as follows:Sn4+ (aq) + 2 e–à Sn2+ (aq) Eº = +0.13 V2 H+ (aq) + 2 e–à H2 (g) Eº = 0.00 V

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1...
Questions



English, 24.09.2019 23:00

Health, 24.09.2019 23:00

English, 24.09.2019 23:00

English, 24.09.2019 23:00

Mathematics, 24.09.2019 23:00

History, 24.09.2019 23:00

Biology, 24.09.2019 23:00



Mathematics, 24.09.2019 23:00

Mathematics, 24.09.2019 23:00


Mathematics, 24.09.2019 23:00

Mathematics, 24.09.2019 23:00




History, 24.09.2019 23:00

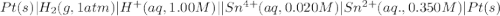
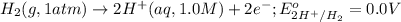
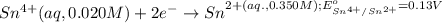
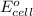 of the reaction, we use the equation:
of the reaction, we use the equation: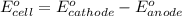
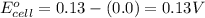
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2[Sn^{2+}]}{[Sn^{4+}]}](/tpl/images/0525/4106/69569.png)
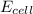 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=1.00M](/tpl/images/0525/4106/641ea.png)
![[Sn^{2+}]=0.350M](/tpl/images/0525/4106/7d5cb.png)
![[Sn^{4+}]=0.020M](/tpl/images/0525/4106/c69f3.png)